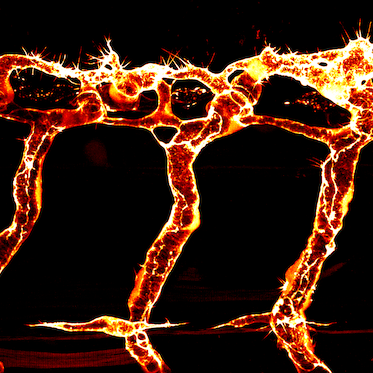
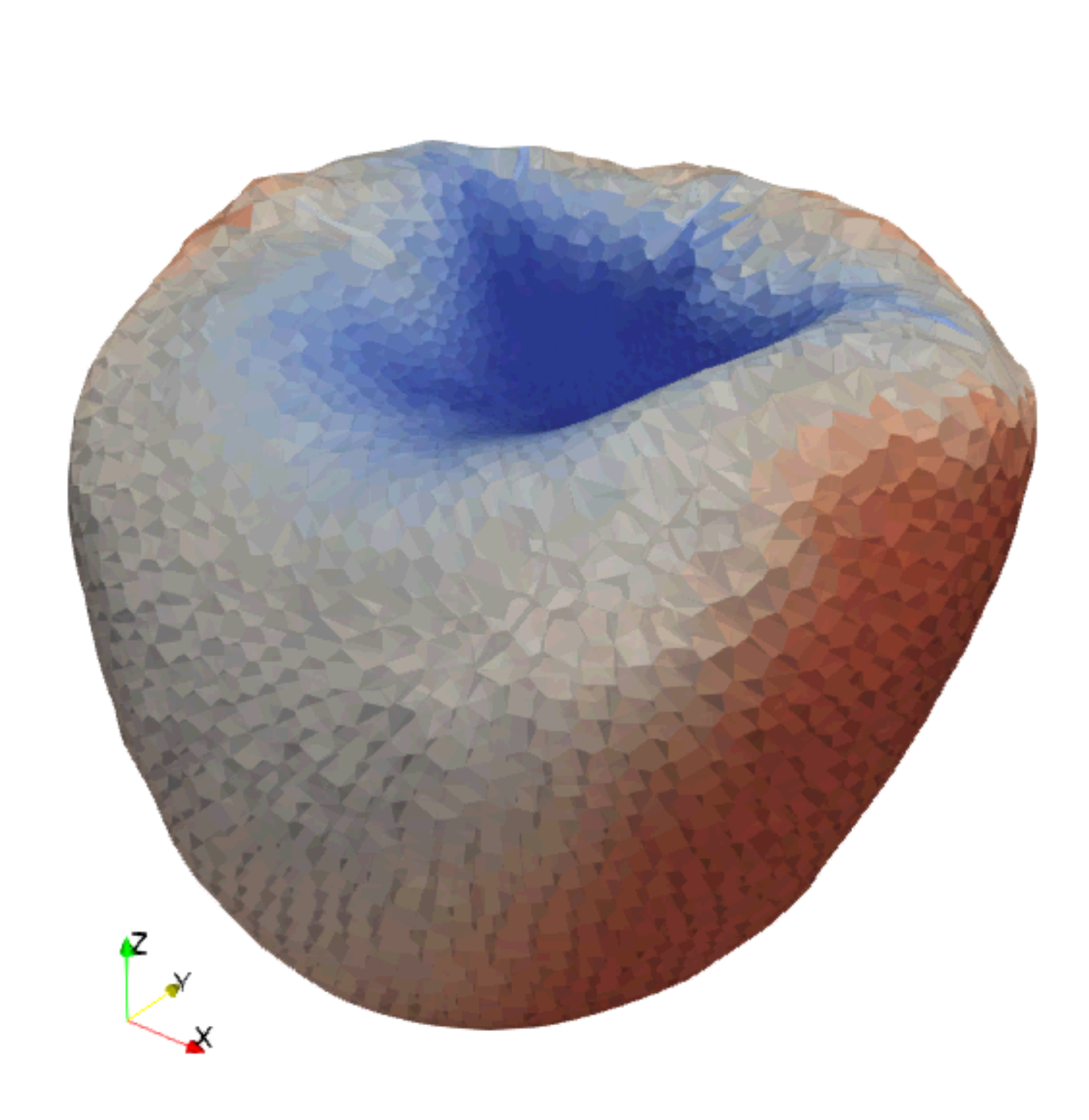
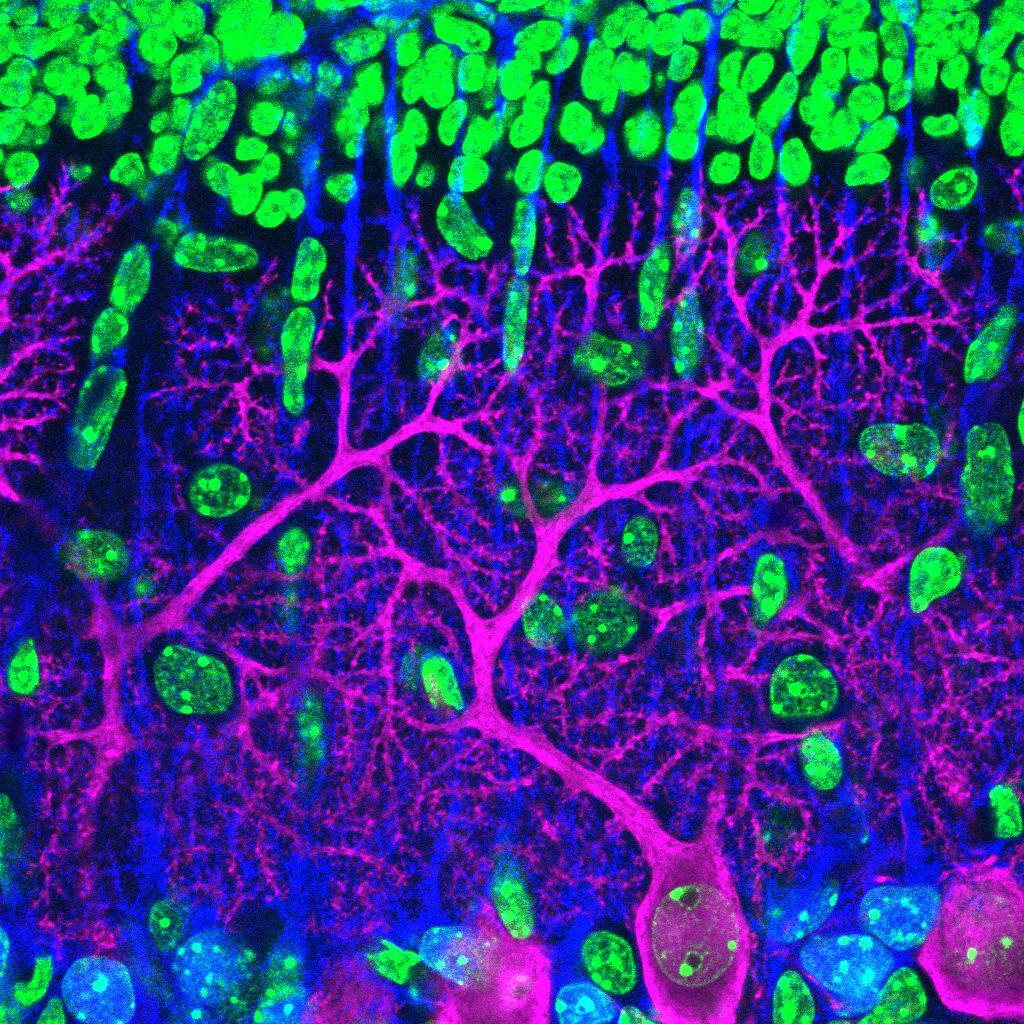
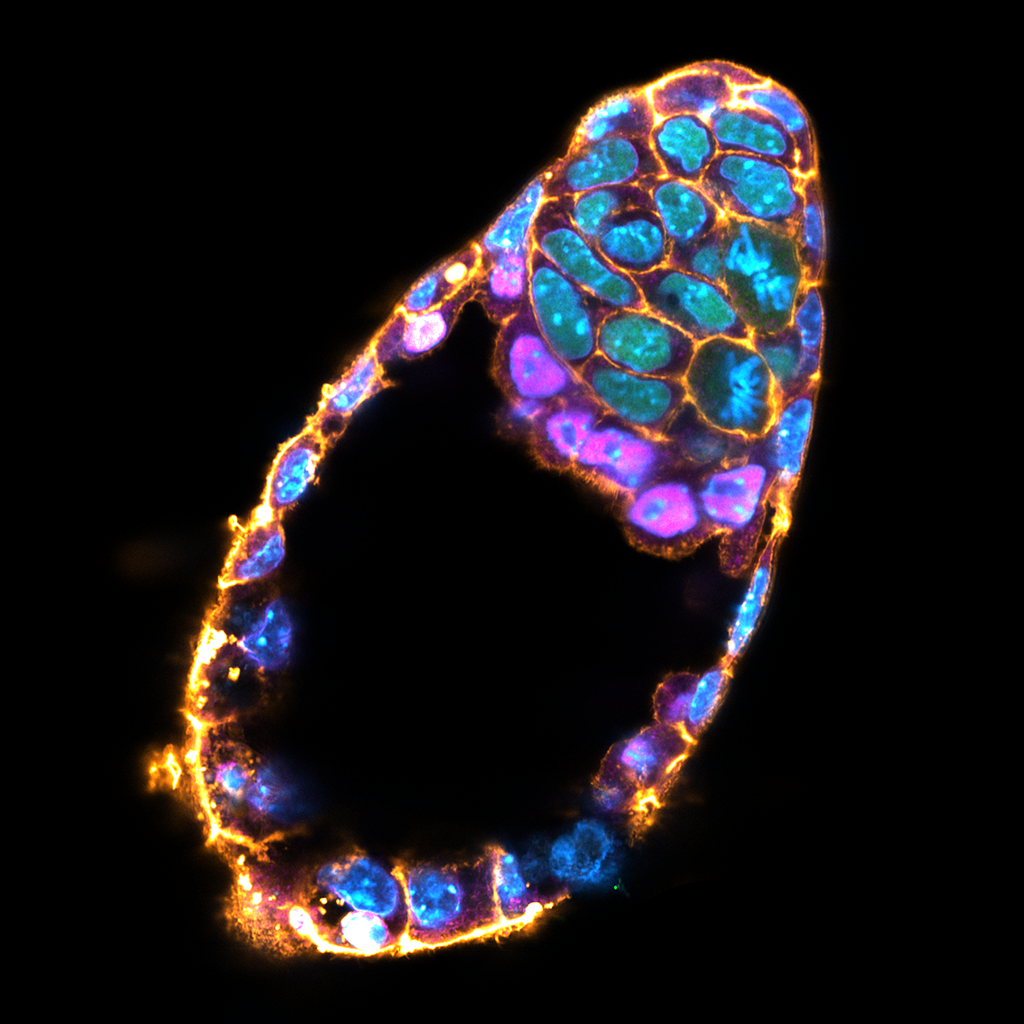
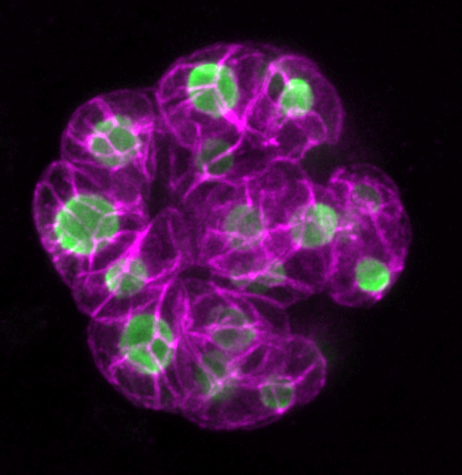
The blue parts show how the group of cells interacts with each other to create an organized pattern, such as embryos and lumens. The orange and blue arrows indicate direction and strength of mechanical forces acting on the cells and the extracellular space, respectively. The gear surrounding the group of cells describes how the balance of complex forces orchestrates the emergent properties of self-organizing multi-cellular system.
Understanding and harnessing the mechanics in multi-cellular systems.
An embryo produces cells with specific fates, forms, and functions during development. These cells are self-organized into an ordered pattern through collective interactions of biomolecules and mechanical forces at various spatio-temporal scales. Through holistic understanding of how mechanical forces elicit self-organizing feedback leading to progressive self-tuning transformation of multicellular systems, we aim at developing new paradigms of the fundamental design principles of biological systems.
With cutting-edge technologies needed to interrogate the mechanical processes, we will establish a unique model for multi-disciplinary research that harnesses expertise from biomedical sciences, engineering, mathematics, physics, and chemistry. Our research projects will advance innovation at the interface of biology and physics, which will create seeds and contribute to social transformation.
Recruitment
News
All
-
Mar. 11, 2024
- Events
Event Date: Jun. 17 〜 Jun. 18, 2024International symposium “Mechanical Control of Biological Self-organisation” on 17-18 June, 2024 at Kyoto (Poster application deadline: 17 May, Registration deadline: 10 June) -
Dec. 15, 2023
- Achievements
A new paper by the A02-1 Shindo team was published in iScience! -
Dec. 4, 2023
- Achievements
A new paper by the A02-1 Phng team was published in PLOS Computational Biology! -
Aug. 28, 2023
- Achievements
A new collaborative work by the B01-2 Yoshimura team and the A03-11 Furutani-Seiki team has been published in FASEB journals! -
Aug. 1, 2023
- Achievements
A new paper by the B01-2 Yoshimura team was published in Nature Communications! -
Jul. 10, 2023
- Achievements
A new paper by the A01-3 Kondo team was published in Cell Reports! -
Jun. 21, 2023
- Events
Event Date: Jun. 15 〜 Jun. 16, 2023The 3rd Transformative (A) RA Team Meeting was held on June 15-16 at Kanazawa Cultural Hall. -
Oct. 18, 2022
- Events
Event Date: Oct. 17 〜 Oct. 18, 2022 The first meeting (kick-off meeting) was held at Hokkaido University.
The first meeting (kick-off meeting) was held at Hokkaido University. -
Sep. 9, 2022
- Events
Event Date: Sep. 9 〜 Sep. 16, 2022Announcement of Publicly Offered Research Online Briefing Session -
Aug. 1, 2022
- Events
Event Date: Aug. 1 〜 Oct. 5, 2022Call for Publicly Offered Research proposal
Events
-
Mar. 11, 2024
- Events
Event Date: Jun. 17 〜 Jun. 18, 2024International symposium “Mechanical Control of Biological Self-organisation” on 17-18 June, 2024 at Kyoto (Poster application deadline: 17 May, Registration deadline: 10 June) -
Jun. 21, 2023
- Events
Event Date: Jun. 15 〜 Jun. 16, 2023The 3rd Transformative (A) RA Team Meeting was held on June 15-16 at Kanazawa Cultural Hall. -
Oct. 18, 2022
- Events
Event Date: Oct. 17 〜 Oct. 18, 2022 The first meeting (kick-off meeting) was held at Hokkaido University.
The first meeting (kick-off meeting) was held at Hokkaido University. -
Sep. 9, 2022
- Events
Event Date: Sep. 9 〜 Sep. 16, 2022Announcement of Publicly Offered Research Online Briefing Session -
Aug. 1, 2022
- Events
Event Date: Aug. 1 〜 Oct. 5, 2022Call for Publicly Offered Research proposal
Achievements
-
Dec. 15, 2023
- Achievements
A new paper by the A02-1 Shindo team was published in iScience! -
Dec. 4, 2023
- Achievements
A new paper by the A02-1 Phng team was published in PLOS Computational Biology! -
Aug. 28, 2023
- Achievements
A new collaborative work by the B01-2 Yoshimura team and the A03-11 Furutani-Seiki team has been published in FASEB journals! -
Aug. 1, 2023
- Achievements
A new paper by the B01-2 Yoshimura team was published in Nature Communications! -
Jul. 10, 2023
- Achievements
A new paper by the A01-3 Kondo team was published in Cell Reports!
Team
A01
Self-organization of multi-cellular systems by mechanical forces within cells
Elucidate how “forces generated by cells” orchestrate cell-to-cell communication in the self-organization process of living organisms.
A02
Self-organization of multi-cellular systems by mechanical forces from extracellular spaces
Elucidate how “forces derived from the extracellular environment” control the emergence of tissue function.
B01
Development of techniques for measurement, manipulation, and analysis of mechanical self-organization
Measure and manipulate forces applied to cell populations and the extracellular environment in living organisms, and quantitatively analyze their spatiotemporal patterns.