Self-organization of multicellular systems by mechanical forces
2025-2026
A03-14
Autonomous maintenance of tissue robustness by cell competition via mechano-chemical crosstalk
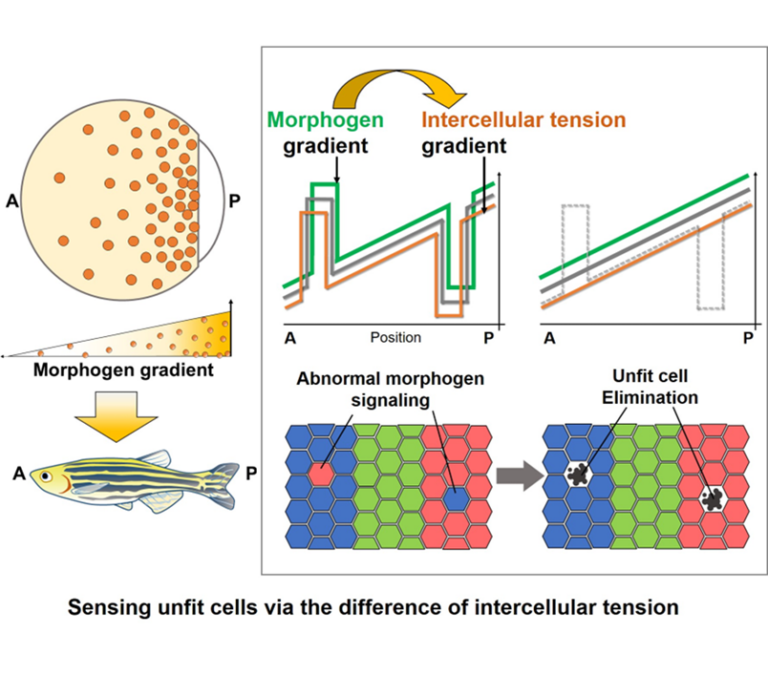
This research aims to understand the mechanisms by which tissues autonomously eliminate abnormal cells which occur naturally via cell competition and maintain robust tissue patterning. Focusing on the mechanical interaction between abnormal cells and neighboring fit cells, we will elucidate a new physicochemical basis for intercellular communication, in which abnormalities in various chemical signals are converted into the change of intercellular mechanical force and sensed by neighboring fit cells.
A03-15
Elucidating the mechanisms underlying cardiac lumen morphogenesis by blood flow-thermal dynamics
Cardiac lumen constantly undergoes physical stresses, such as blood flow and myocardial contraction, from the heart morphogenesis. Our studies have identified that mechano-responsive signals happen in both spatial and temporal manner during the cardiac lumen formation. However, the mechanism by which the cells appropriately regulate mechanical signals in response to the continuous forces is still unclear. In this project, we aim to elucidate how the structured cardiac lumen is organized for functional blood circulation, focusing on the “forces generated from blood flow” and “biological signals” during the lumen formation, and how cells discriminate the characteristics of forces. Specifically, we develop a technique that directly manipulates physical forces in zebrafish embryos and evaluate the biological signal outputs by quantitative approaches.
A03-16
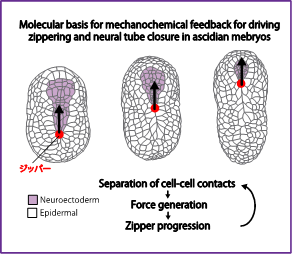
Mehcano-chemical feedback mechanisms underlying zippering and neural tube closure in a simple chordate
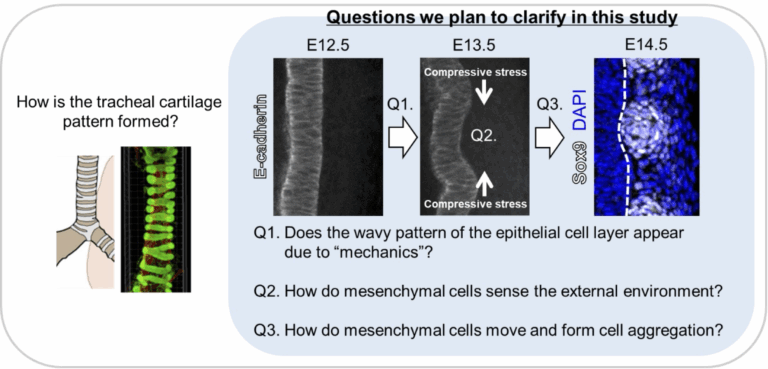
Terrestrial animals with lungs continuously maintain respiration while performing various movements. The ability to keep the airway open even when the neck or body bends is due to the patterned structure of tracheal cartilage, which resembles a bellows. Structurally, the trachea consists of an inner epithelial layer and an outer mesenchymal layer comprising cartilage, ligaments, and smooth muscle. Our previous analyses indicate that interactions between these two layers are important for proper tracheal morphogenesis during development. We also found that the epithelial layer undergoes a transient deformation, which appears to play a role in tracheal morphogenesis. Notably, this deformation is conserved across species, as similar patterns are observed not only in mammals (mouse) but also in Aves (chicken) and reptiles (gecko), which also possess patterned tracheal cartilage. In this study, we aim to clarify the mechanical interactions between epithelial and mesenchymal tissues in the embryonic trachea using mouse, chick, and gecko embryos as model. Through this approach, we seek to elucidate the underlying principles of tracheal cartilage pattern formation and to determine whether these mechanisms are evolutionarily conserved.
A03-17
Molecular basis for mehcano-chemical feedback underlying sequential contraction during zippering and neural tube closure in a simple chordate.
Self-organizing patterns of multicellular movements emerge from the dynamic interplaying of biochemical signaling and force generation, shaping tissues and organs. Our recent research suggests that separation of cell-cell contact induces activation of actomyosin contractility for zippering and neural tube closure in ascidian embryos. In this team of the Transformative (A) research area, I aim to elucidate the mechanism by which the separation of cell-cell contacts regulate the activation of actomyosin contractility during ascidian neural tube closure.
A03-18
Autonomous growth mechanism of luminal structures through dynamic regulation of epithelial barrier function
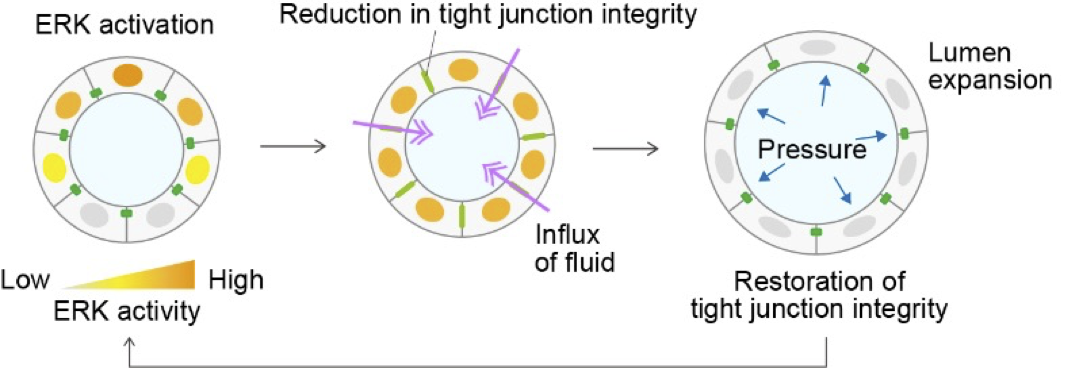
Our research focuses on the dynamic regulation of tight junctions in epithelial tissues. We have shown that ZO-1, a scaffold protein of tight junctions, translocates to basal podosome-like structures in response to ERK signaling during collective migration, where it contributes to mechanical force generation and ECM remodeling. This finding led us to a broader question: how does the redistribution of junctional proteins affect epithelial barrier function and morphogenesis? In our upcoming studies, we aim to investigate how ERK activity and ZO-1 localization dynamically interact to control tight junction formation and lumen development.
A03-19
Elucidating the Mechanism and Forces Driving Body Axis Rotation During Organogenesis
Since multiple organs are involved in the shaping of the entire embryo during the organogenesis phase, the understanding of mechano-developmental mechanism is still unknown. The ascidian provides an interesting model to investigate how interactions among multiple tissues or organs contribute to the evolutionary conserved embryo shape in chordates. This study aims to elucidate the mechanism of ascidian tailbud embryo morphogenesis at trans-scale level, with a focus on body axis rotation observed in chordate embryos during the organogenesis period.
A03-20
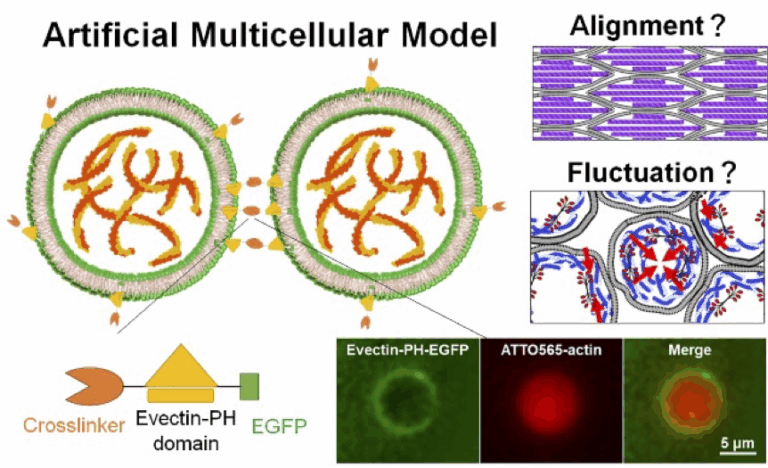
Elucidation of the Hierarchical Synchronization Mechanism of the Cytoskeleton through Mechanical Interactions
The cytoskeleton drives force generation, morphogenesis, and mechanosensing in cells, but its contribution to macroscopic tissue organization remains largely unclear. This study elucidates the mechanism by which the cytoskeleton’s dynamic behavior (such as fluctuations or changes in alignment) is spatiotemporally modulated beyond the single-cell level, using a reconstructed multicellular model to examine mechanical interactions between artificial cell units.
A03-21
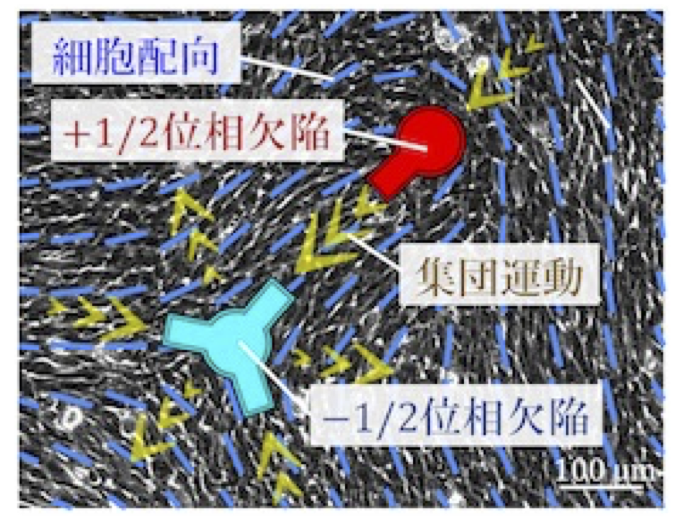
Mechanical understanding of multicellular ordering and morphogenesis from the physics of active nematics
Cells often exhibit collective motion within multicellular groups, and the orientational order of an aligned cell population is a primary factor in determining multicellular morphology. Within these collectives, geometric singular points called topological defects exist. These defects induce regular patterns of cellular flow and, in some cases, trap or deplete cells around the defects. However, the mechanism by which defects are geometrically controlled remains elusive. In this study, we develop a new technique to control the spatial arrangement of topological defects in an active nematic cell population. The goal of this project is to elucidate the principle by which the regularity of defect positioning governs tissue morphology.
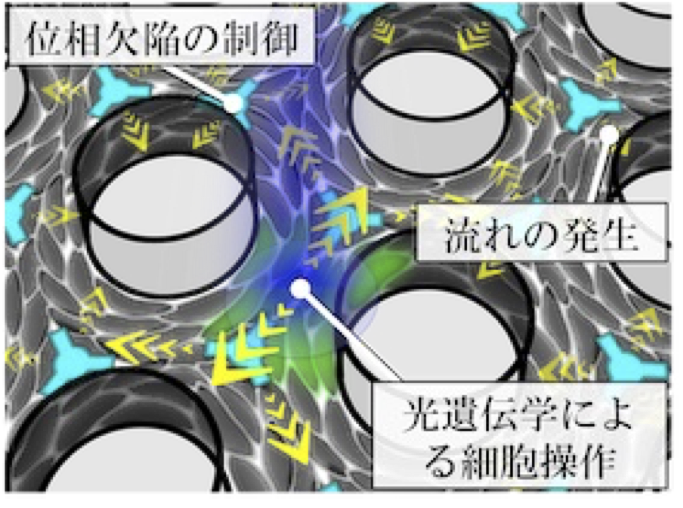
A03-22
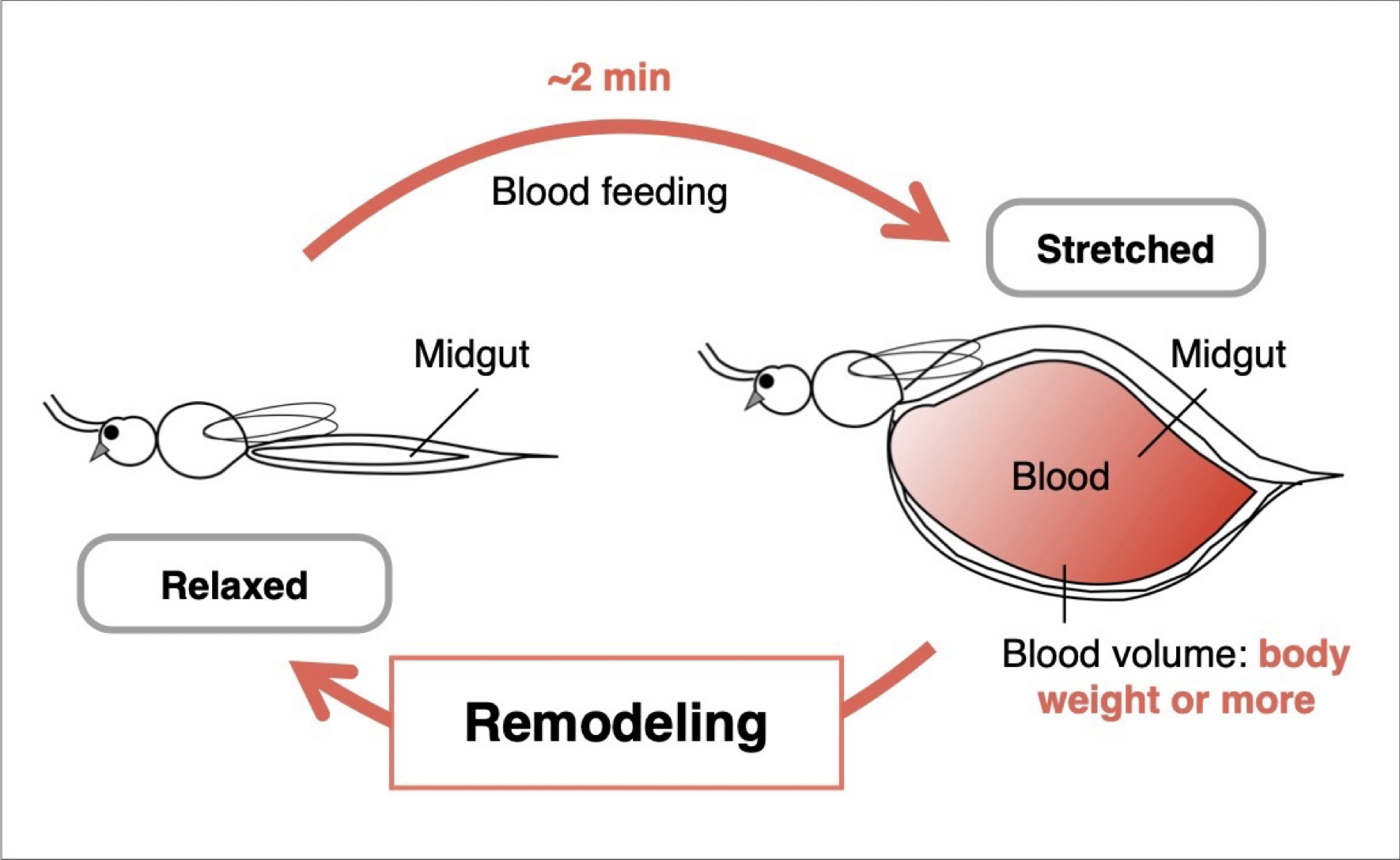
Midgut remodeling induced by mechanical stimuli from blood feeding in mosquitoes
Mosquitoes, which are blood-feeding arthropods, can ingest an amount of blood equivalent to or even exceeding their own body weight within just two minutes. During this process, the midgut undergoes rapid expansion, followed by gradual contraction as digestion and absorption of the blood proceed. These dynamic structural changes in the midgut are not merely physical deformations, but can be regarded as an orchestrated tissue remodeling process that leads to the formation of new structural organization. In this study, we focus on the remodeling of the mosquito midgut that occurs after it becomes fully engorged, aiming to elucidate the mechanisms of blood-feeding-induced mechanosensory responses in midgut epithelial cells.
A03-23
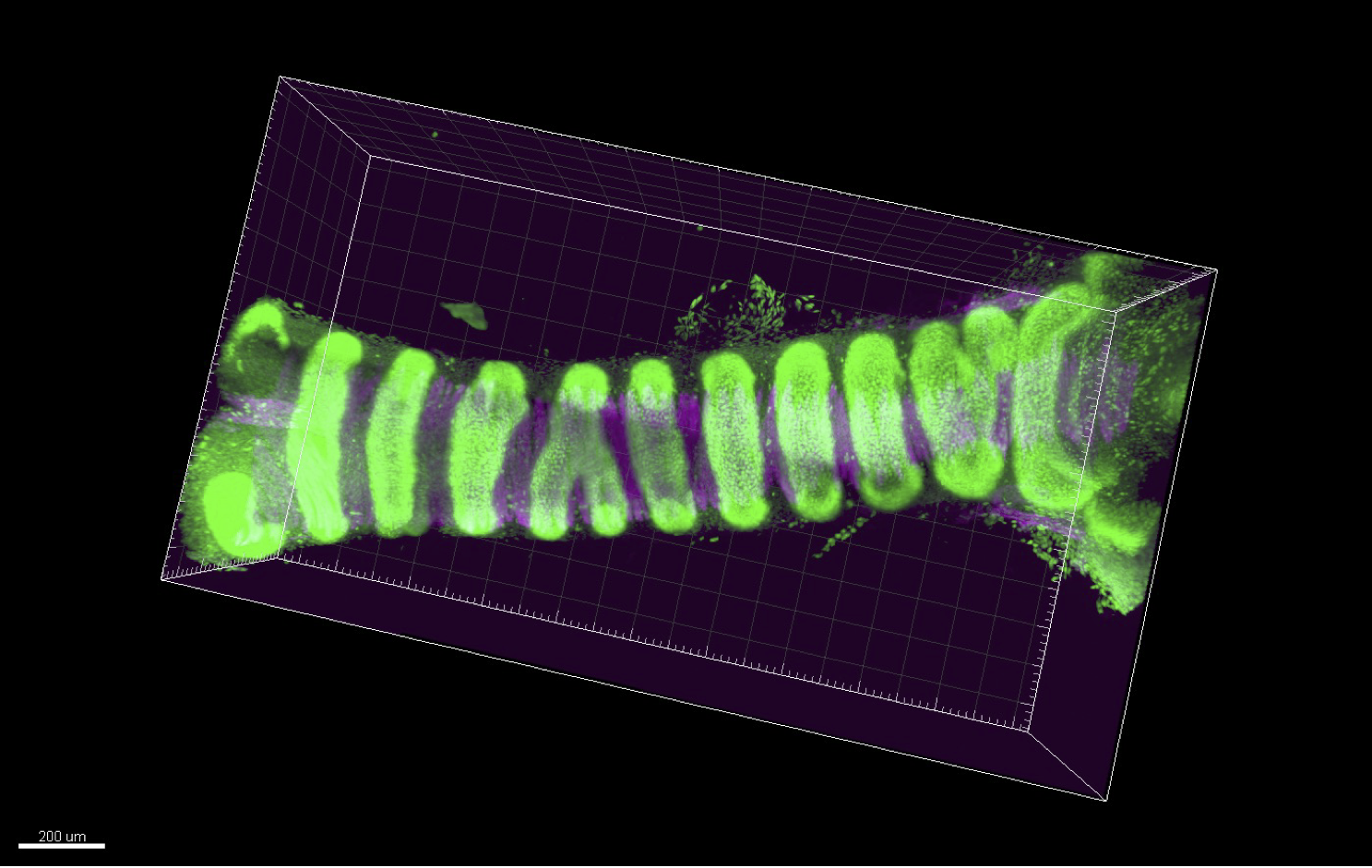
Self-organized forces generated through Cell–ECM interactions drive the emergence of periodic tissue morphology
Our laboratory investigates the development and regeneration of the respiratory system. In this project, we focus on the trachea that is a large tubular organ directing inhaled air from the oral cavity to the lungs. The trachea is surrounded by C-shaped cartilage rings that not only provide structural stiffness to maintain luminal patency but also contribute to flexibility through their periodic arrangement. Precise morphogenesis of the periodic ring structures is essential as its malformations can lead to severe congenital disorders. The previous developmental biology studies have primarily focused on epithelial deformation as the driving force behind organ-specific morphogenesis. However, recent research has revealed that the mesenchymal tissue also plays a crucial role in organ morphogenesis. In this project, we investigate the periodic cartilage patterning of the mammalian trachea as a model to test the hypothesis that mechanical forces generated through Cell–ECM interactions regulate the self-organization of periodic cartilage structures. In our previous work, we identified subpopulations of chondroprogenitor cells. We aim to validate that periodic condensation of cartilage cells is self-organized through cell migration. This will be achieved by integrating mouse genetics, ex vivo fetal trachea cultures, differentiation of pluripotent stem cells, and dynamic analysis using micro-engineered devices.
A03-24
()
()
A03-25
Tissue elongation and the underlying mechano-effectors in cellular slime molds
Tissue self-organization in motile cell populations involves a coordination of forces acting between cells and other substrates. These dynamics are prevalent in Metazoa, however there are also some cases outside Metazoa including the Amoebozoa species cellular slime molds that have apparently acquired similar dynamics. In this study, we focus on the mechanical basis of their organization by analyzing the dynamics of tissue elongation and cell rearrangement and stress distribution at the tissue level.

A03-26
()
A03-27
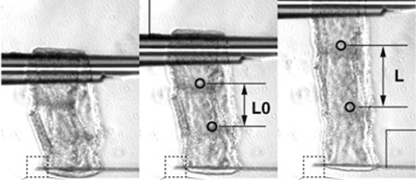
Elucidation of diversity and universality of elongation mechanisms of protruding organs using limb buds and tails buds
The purpose of this research is to test the hypothesis that the elongation of protruding organs, such as limb buds, follows a universal developmental principle: “The entire organ elongates unidirectionally toward the distal side due to mechanical constraints imposed by epithelial tissue oriented perpendicular to the elongation axis, which restrict the mesenchyme’s expansion to that direction.” Specifically, we will use the elongation processes of the chick embryo’s limb buds and tail as model systems to investigate the diversity and universality of morphogenetic mechanisms in protruding organs from the following five perspectives: 1. Epithelial tissue responsiveness: To determine whether epithelial tissues respond to internal pressure from mesenchymal cells and influence cell orientation, we will analyze the response of epithelial tissues when the shape of the mesenchymal tissue is artificially deformed. This will be achieved by injecting cells into the mesenchyme to modify internal pressure. 2. Mesenchymal tissue responsiveness:To examine how mesenchymal cell populations change their organization (i.e., anisotropy) under different mechanical conditions, we will isolate limb bud mesenchyme and culture it under artificial compression along axes other than the anteroposterior axis. 3. Universality of morphogenetic mechanisms:To assess whether the observed cellular responses are organ-specific or universal, we will conduct parallel experiments using the tail tissue of chick embryos. 4. Autonomous shape-determination mechanisms:To investigate whether the initial organ shape autonomously determines epithelial cell orientation, we will perform stress simulations to analyze the internal stress distribution within the epithelial tissue during early development. 5. Three-dimensional assessment of mesenchymal pressure:To directly evaluate the directionality of internal mesenchymal pressure, we will inject fluorescent droplets and observe their deformation in three dimensions. Through this study, we aim to redefine the traditional concept of tissue interaction by incorporating mechanical force exchange between epithelial and mesenchymal tissues. We propose a new concept in developmental biology: that protruding organs undergo autonomous morphogenesis through force-mediated interactions between these tissues.
2023-2024
A03-1
Autonomous maintenance of tissue robustness by cell competition via mechano-chemical crosstalk
This research aims to understand the mechanisms by which tissues autonomously eliminate abnormal cells which occur naturally via cell competition and maintain robust tissue patterning. Focusing on the mechanical interaction between abnormal cells and neighboring fit cells, we will elucidate a new physicochemical basis for intercellular communication, in which abnormalities in various chemical signals are converted into the change of intercellular mechanical force and sensed by neighboring fit cells.

A03-2
Elucidating the mechanisms underlying cardiac lumen morphogenesis by blood flow forces
Cardiac lumen constantly undergoes physical stresses, such as blood flow and myocardial contraction, from the heart morphogenesis. Our studies have identified that mechano-responsive signals happen in both spatial and temporal manner during the cardiac lumen formation. However, the mechanism by which the cells appropriately regulate mechanical signals in response to the continuous forces is still unclear. In this project, we aim to elucidate how the structured cardiac lumen is organized for functional blood circulation, focusing on the “forces generated from blood flow” and “biological signals” during the lumen formation, and how cells discriminate the characteristics of forces. Specifically, we develop a technique that directly manipulates blood flow forces in zebrafish embryos and evaluate the biological signal outputs in response to forces by the quantitative imaging approach.

A03-3
Mehcano-chemical feedback mechanisms underlying zippering and neural tube closure in a simple chordate
My research seeks to address how self-organized collective cell movements emerge through the dynamic interplay of cell-cell signaling, force generation and tissue remodeling during embryonic development. Ascidians are marine invertebrate chordates, that are the closest relatives of vertebrates. They provide the opportunity to both manipulate and observe the dynamics of signaling, cell contractility and adhesion from subcellular to tissue scales, in the context of simple and highly stereotyped morphogenetic events with very few large cells in optically clear embryos. Our recent results suggest that a separation of cell-cell contact induce activation of actomyosin contractility for zippering and neural tube closure in ascidian embryos. In this team of the Transformative (A) research area, I will elucidate how the separation of cell-cell contacts is translated into activation of actomyosin contractility during ascidian neural tube closure.

A03-4
Elucidation of Self-Transformation and Forces that Control Body Axis Rotation of Tailbud Embryo
Since multiple organs are involved in the shaping of the entire embryo during the organogenesis phase, the understanding of mechano-developmental mechanism is still unknown. The ascidian provides an interesting model to investigate how interactions among multiple tissues or organs contribute to the evolutionary conserved embryo shape in chordates. This study aims to elucidate the mechanism of ascidian tailbud embryo morphogenesis at trans-scale level, with a focus on body axis rotation observed in chordate embryos during the organogenesis period.

A03-5
Long-range mechanical signals that regulate organogenesis
Peristaltic waves, contractile waves of smooth muscles, propagate along gut tube over long distances, which potentially act as long-range mechanical signals. Using optogenetics and live imaging in chicken embryonic gut I investigate the mechanism in which the peristaltic waves regulate gut organogenesis such as gut tube elongation and villi formation. Through this project, I aim to propose the fundamental principle for accounting macro-scale morphogenesis.
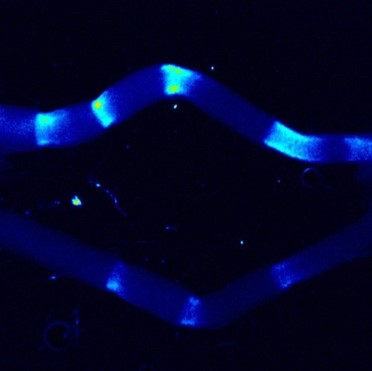
A03-6
Active gel physics for self-organized patterns and mechanochemical cross-talk in confined cytoskeletal systems
The contractile forces exerted by the actomyosin cytoskeleton can influence intracellular symmetry and non-equilibrium dynamics, ranging from single cells to biological tissues. However, bridging the microscopic forces to the macroscopic cellular ordering remains a significant challenge in the field of active matter physics. In this study, we investigate the physics of active actomyosin gels to elucidate the mechanisms underlying force-induced structural organization. Furthermore, we aim to extend this approach to a theoretical model of cell populations, shedding light on the principles of force-mediated collective ordering.
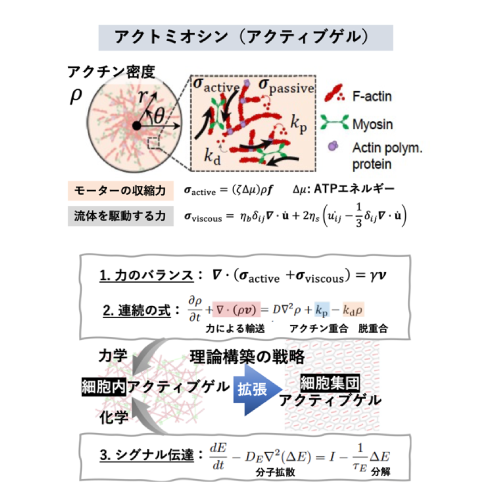
A03-7
Molecular coordination mechanisms between mechanical forces and chemical reaction during mammalian neural tube closure
Our group has studied mechanisms of mouse neural tube closure; in particular the role of neural folds which are located between neural ectoderm and surface ectoderm. Before the closure, these cells in the neural folds have initially uncommitted characters in which neither neural nor surface ectoderm markers are expressed but stem cell markers are. Grainyhead-like (GRHL)3 protein expresses specifically in these uncommitted progenitors in the nucleus and directs epidermal differentiation by transactivating epidermis-specific genes such as E-cadherin under the control of canonical Wnt signaling. Upon epidermal differentiation, the GRHL3 protein is localized in the cytoplasm and activates non-canonical Wnt signaling. Thereby, the cellular architecture of the epidermis has highly enriched actomyosin networks and the cell cortex becomes much stiffer. This project aims to understand how GRHL3-positive neural-fold cells sense mechanical forces within neural and surface epithelial sheets and integrate them into two major cellular biochemical signaling pathways at the molecular level during mammalian neural tube closure.
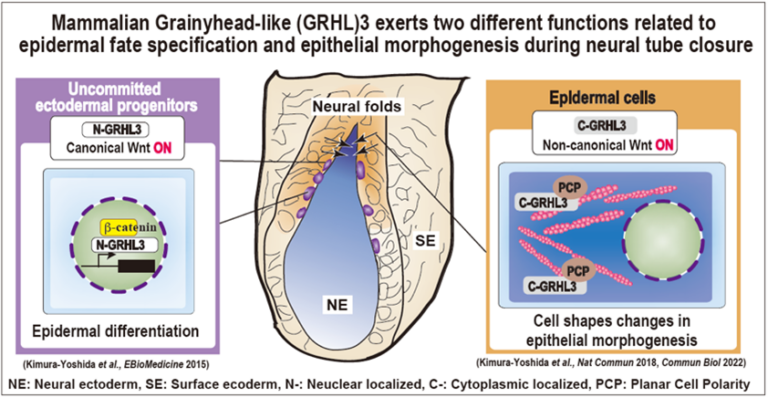
A03-8
Relationship between cellular mechanosensation and polarized cell/tissue dynamics during organ morphogenesis
In this study, we aim to clarify the connection between cellular mechanosensation and polarized cell/tissue dynamics, which plays a critical role in shaping organ-specific morphology. Specifically, we are focusing on the early developmental stages of the forebrain and heart to uncover shared morphogenetic mechanisms between organs.
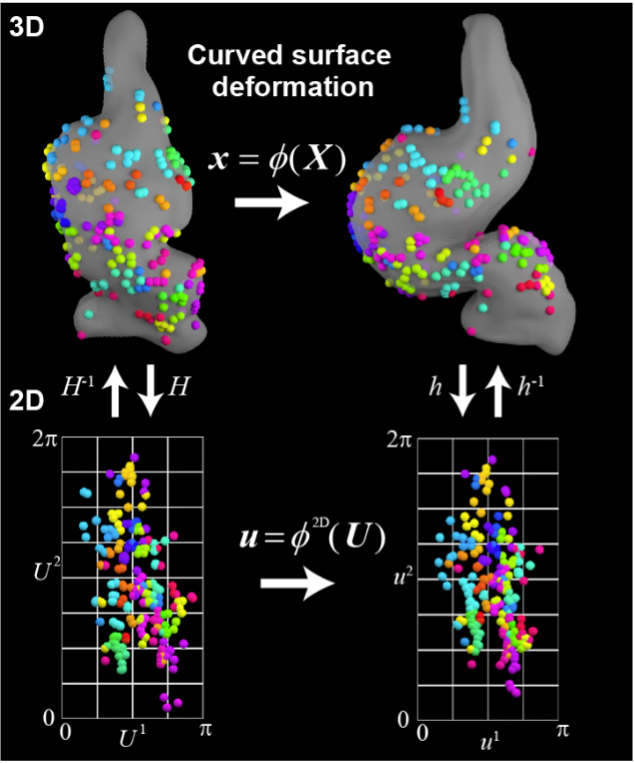
A03-9
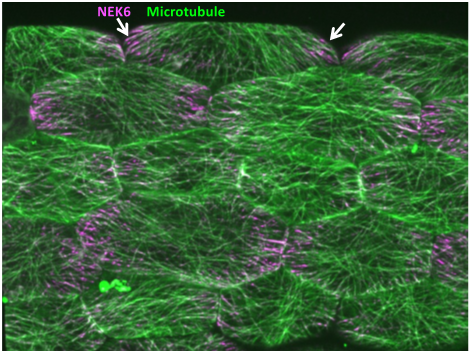
Mechanical feedback system that allows for directional growth and posture control in plants
Morphogenesis inevitably involves instability because of the constant changes in shape and size. To cope with this constant spatiotemporal change and to ensure robust organogenesis, the proprioception mechanism is required for the recognition of the relative position of each body part and to provide a feedback to the overall shape. In plants, mechanical feedback has been proposed to coordinate the growth of individual cells and the entire organ. In this feedback, tension generated during morphogenesis serves as a signal and changes the orientation of microtubules, but the overall mechanism is still unknown. Here, we will elucidate the mechanism of microtubule alignment to the tensile stress, which is the key to mechanical feedback. My laboratory has previously found a NIMA-related kinase that has a unique property of changing its subcellular localization in response to tensile force. We will use the NIMA-related kinase as a clue for imaging the tension response and analyzing the mechanism of mechanical feedback. We will also search for novel factors that regulate the mechanical feedback.
A03-10
Investigating the mechanical-chemical crosstalk that controls organismal reorganization in basal metazoans
This research aims to elucidate the nature of the mechanochemical crosstalk and self-organization mechanisms that enable organismal-level regeneration, or reorganization, in basal metazoans, using the jellyfish Cladonema. I will investigate the mechanisms of pattern formation during organismal reorganization at cellular and molecular levels and pursue the universal principles of self-organization that can be common to higher animals.

A03-11
The mechanical control of tissue regeneration by YAP mechano-homeostasis
When a part of the organ is lost, how do cells in an organ sense the whole organ size, proliferate and differentiate to restore the original organ size? How do cells stop doing so when the original organ size is restored by regeneration?
Our analysis of the “hirame” medaka fish mutant whose body and organs become flat due to its inability to withstand external forces uncovered the mechanism by which YAP generates an 3D organ that withstand external forces. YAP mediated cell tension control coordinates 3D tissue formation by cell stacking and subsequent 3D tissue alignment for generating a complex organ and the 3D body shape. The latter is mediated by YAP through the bidirectional mechanical control between a cell and extracellular matrix (ECM), which maintains tissue homeostasis (therefore, we named it YAP-mechanohomeostasis: this is the mechanism “You push me, I push you back.”).
Since YAP is required for regeneration, we aim to uncover how YAP-mechano-homeostasis contributes to regeneration by analyzing dynamics of YAP activation, ECM mechanics and mechanical response during regeneration in this study.
A03-12
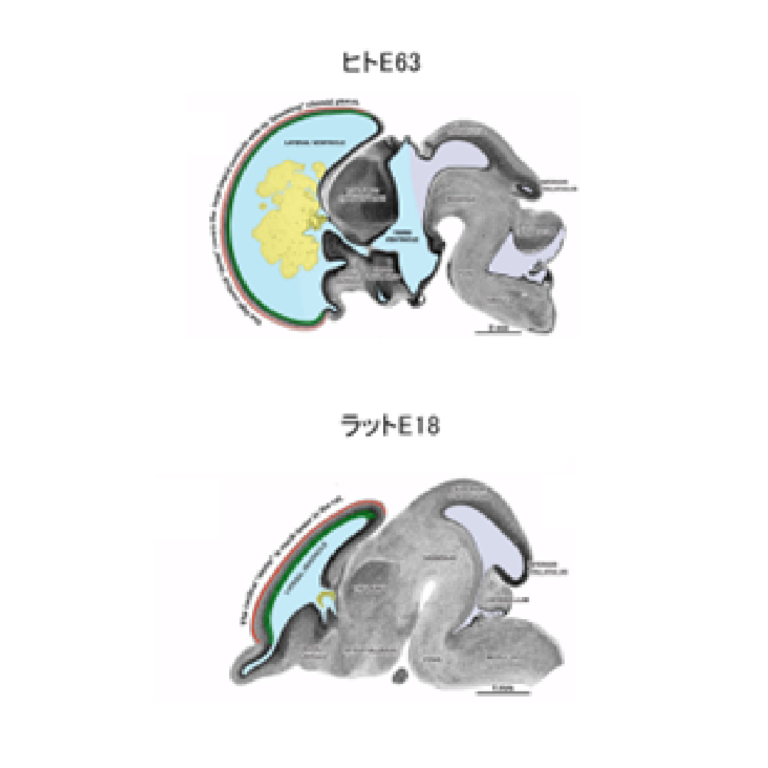
Roles for intraventricular pressure in brain development
We explore roles for internal pressure of the brain ventricles mediated by cerebrospinal fluid (CSF) in brain development. We particularly focus on the transition from proliferative to neurogenic mode of the neural progenitor cells and on the expansion of the neural progenitor pool. Our research includes development of a method to measure the intraventricular pressure of different vertebrate species. We hope that notions obtained will also provide insights into brain evolution, disease, and organoid technologies.
A03-13
Elucidation of the elongation mechanism of the entire limb bud by analysis of force-tissue interaction
Our research uses the limb bud of the chick embryo as a model system and analyzes the force interaction between the epithelium and mesenchyme of the limb bud, which are different tissues. We aim to elucidate the “mechanism by which the organs of the entire limb bud extend autonomously along the distance axis”. In our previous research, we found that the mechanical stress is five times higher along the anteroposterior axis, which is perpendicular to the elongation direction, and that there is stress anisotropy inside the epithelial tissue of the limb bud. Therefore, in this research, we will clarify the above from the following two perspectives, based on macro and micro level. (1) Macroscopic view: Using tissue culture, spatio-temporal quantitative analysis of the stress inside the epithelial tissue of the limb bud and measurement of the physical properties (Young’s modulus) of the epithelial tissue revealed compressive force to the entire mesenchymal cells inside. (2) Microscopic perspective: In order to understand the relationship between force and cell response and the mechanism that generates force at the cellular level, the cellular dynamics of epithelial tissue that produces stress anisotropy, the localization of molecules responsible for force. We will analyze changes in the order of cells in the mesenchyme (anisotropy of cell populations) at the micro level. In the field of developmental biology, research on the elongation mechanism of limb buds has been conducted mainly based on the expression of secretory factors, the tissue interaction between the epithelium and mesenchyme, and the analysis of the genes involved in it. Through this research, the concept of inter-tissue interaction has been reconsidered and redefined as “inter-tissue interaction including force exchange between the epithelium and mesenchyme”, and by redefining it, the entire macroscopic limb bud elongation mechanism will be clarified.





















